liver tumor
Under construction
Under construction
Under construction
Male in the 40s
Chief Complaint: No significant symptoms
Medical History: Not significant
Current Medical History:
He was diagnosed with liver cancer in November 2010. After he began chemotherapy in December the same year, he had suffered from side effects from chemotherapy, including body pain, general fatigue and appetite loss. For the reasons, he began Fucoidan Mix AG therapy in February 2011.
Treatment Outcome:
AFP levels were decreased from 85.83 to 58.0, and the side effects were decreased. He was able to get back to work, and his QOL improved.
※Data taken over the period of treatment
【Tumor markers】
Fucoidan daily intake per day: Approximately 3.5g
| February 2011 | February 2011 | July 2011 | ||
|---|---|---|---|---|
| AFP (< 10.0) | 85.83 | 77.52 | 58.0 | (ng/ml) |
Data provided by Matsuzaki Memorial Hospital
Female in the 60s
Chief Complaint: No significant symptoms
Past Medical History: Depression
Current Medical History:
She had received treatment for hepatitis B at another hospital for about 20 years. She had been intravenously given 5 ampoules of conservative treatment of liver sick drug (SMC) four days a week, however no improvement had been observed. She visited our clinic in August 2002. After the informed consent procedure, she began taking Fucoidan Mix AG as a treatment option without impairing the QOL.
Treatment outcomes:
After taking 1.0g of Fucoidan Mix AG per day, her levels of GOT were reduced to 9450, and her GPT levels were reduced to 79 from 36. She had received 5 ampoules of liver asylum agent (SMC) (intravenous injection) at the previous hospital. During the treatment at our hospital, the agent was reduced to 1 ampoule from 3 ampoules, then administration of SMC became no longer necessary 6 months later.
※Data taken over the period of treatment
【Biochemistry tests】
Fucoidan Mix AG intake per day: Approximately 1.0g
| May 2002 | August 2002 | October 2002 | March 2003 | June 2004 | ||
|---|---|---|---|---|---|---|
| GOT (10〜40) |
107 | 94 | 79 | 45 | 50 | (IU/l) |
| GPT (5〜40) |
115 | 79 | 66 | 37 | 36 | (IU/l) |
SMC 5A→3A→1A→off(Half a year later)
Female in the 60s
Chief Complaint: No significant symptoms
Past Medical History: Angina pectoris
Current Medical History:
She had received treatment for hepatitis C at another hospital. She visited our clinic in March 2003. After the informed consent procedure, she began treatment with Fucoidan Mix AG.
Treatment outcomes:
After taking approximately 1g of Fucoidan Mix AG per day, her levels of AFP were decreased to 12.0 from 33.4 in approximately 2 months.
※Data taken over the period of treatment
【Tumor markers】
| March 2003 | May 2003 | November 2004 | ||
|---|---|---|---|---|
| AFP (< 10.0) | 33.4 | 23.6 | 12.0 | (ng/ml) |
Male in the 70s
Chief Complaint: General fatigue
Past Medical History: No significant diseases
Current Medical History:
He visited a nearby hospital complaining of, primarily, general fatigue around March 2004, and was given the diagnosis of hepatocellular carcinoma and hepatitis C. He was proposed a surgery, hepatic artery embolization (TAE) or intra-arterial injection therapy, but he was against any of them. He visited our clinic to seek a second opinion as well as information on fucoidan. A detailed examination result indicated the same diagnosis, and we also proposed a surgery, TAE or intra-arterial injection therapy. The patient and his family members opted for fucoidan treatment, so we provided them with sufficient information on fucoidan, and began a mono-therapy of Fucoidan Mix AG.
Treatment Outcome:
Two month after taking Fucoidan Mix AG, his GOT / GPT · PIVKA-Ⅱ levels drastically reduced. 7 months later, which was January 2005, CT images showed that his tumor size had been maintained, and his liver had functioned normally, the condition of which was ranked Long NC (Long No Change). Because he hoped more tumor reduction, we increased the Fucoidan Mix AG daily dosage to approximately 4.0g. After that, the tumor was reduced markedly, the condition of which was classified as PR (Partial Response)
※Data taken over the period of treatment
《CT images of the abdomen》
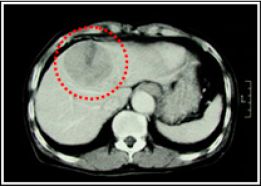
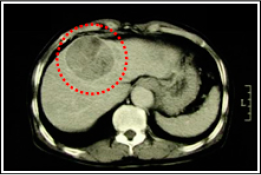
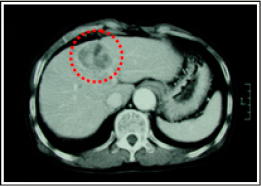
【Tumor markers】
| April 2004 | June 2004 | July 2005 | ||
|---|---|---|---|---|
| AFP (< 10.0) | 20.3 | 44.6 | (ng/ml) | |
| PIVKA-Ⅱ(< 35.0) | 8430 | 1600 | 119 | (mAU/ml) |
【Biochemical tests】
| April 2004 | June 2004 | July 2005 | ||
|---|---|---|---|---|
| GOT/GPT(10~40/5~40) | 135/214 | 49/68 | 35/57 | (IU/ml) |
| γ-GTP(< 70) | 112 | 77 | 78 | (IU/ml) |
Data provided by Matsuzaki Memorial Hospital
Fucoidan Mix AG・・・It is composed of mozuku extracted fucoidan, mekabu extracted fucoidan and agaricus mycelium.
Today, active research is conducted on fucoidan and various bioactive functions of fucoidan, such as “anti-cancer action,” “cholesterol-lowering action,” “blood-pressure lowering action” and “anti-virus action,” have been revealed.